Team:CIDEB-UANL Mexico/project capture
From 2014hs.igem.org
Capture Module

As a step to desalinize water, iGEM CIDEB 2014's project intended to capture sodium ions from saline water using a protein produced from the NhaS gene expression. This gene was obtained from a patent from Krulwich & Ivey from 1992 and no further information was found about the gene in other sources. A lot of research and predictions in different sites had to be made in order to obtain more information about it and being able to work with and include it in the project.
Description
NhaS is a putative protein from Bacillus firmus that is characterized by its ability to bind and sequestering sodium ions, with a calculated weight of 7100 Daltons and a pH of 12. It “can enhance the Na+ -resistance of antiporter- deficient strains by increasing the availability of Na+ to the integral membrane antiporters on the cytoplasmic side of the membrane and by sequestering Na+ while rate-limiting efflux mechanisms catalyze extrusion of the cation.” (Krulwich & Ivey, 1992)
Research by Krulwich and Ivey (1992) supports that in its origin bacteria, NhaS works as a regulation pH homeostasis protein because it makes the cytoplasmic pH more acidic than the external medium, usually basic.
Essentially, NhaS performs three different functions; (1) capturing sodium ions, (2) regulating pH, and (3) enhancing the resistance of bacteria to high saline conditions.

Figure 1. Patent US 5346815 A shows extracts of the E. coli EP432 transformed with pGEM (fig. 4A) and pGRVH (fig. 4B). pGEM is a control plasmid and pGRVH is a plasmid with the NhaS gene. Those are crude extracts shown by the effect of putting the bacteria to an SFBI excitation, which is a sodium-sensitive molecule used to measure intracellular Na+. Resuming, it shows in basic draws that the protein is expressed in E. coli and in what quantity according to the excitation level where it is exposed.
Research on NhaS
It is important to be familiarized with what it is being worked with, and since this putative gene has never been used at iGEM before, it was done a lot of research on it.
The composition and form of a protein show relevant data about its actions and functions, that it is why it was investigated NhaS’ predicted type. It was found in the modelling tool 3D-JIGSAW from Cancer Research UK' site that its possible protein or peptide type would be helix, coil or strand (Fig. 2). Also, it was found in the Raptor X protein modelling site a predicted structure of the protein. (Fig. 3)

Figure 2. Interactive 3D-JIGSAW's result that indicates the predicted protein type of NhaS.

Figure 3. Predicted protein structural results from Raptor X.
Research was done in different sources. The previous information was confirmed in Predict Protein site.

Figure 3. Results given by Predict Protein showing the secondary structure composition and solvent accessibility of the putative NhaS gene.

Figure 4. Results given by Predict Protein showing the predicted precise structure of the NhaS protein.
As it can be apprecciated, the results from all sources match, so, based on the previous information it was concluded that NhaS is most possible to be of the helix type. Being aware of the secondary structure of proteins is relevant, since hence, the protein folding mechanism can be taken into consideration.
According to Krulwich and Ivey (1992), the location of the protein is in the cytoplasmic side of the membrane, however, when the research was made, it was found out that the protein is predicted to be highly non-cytoplasmic (Stockholm Bioinformatics Center SBC Phobius, 2014). Based on this, the team came out with a hypothesis in which NhaS would be located in the inner part of the membrane but on its cytoplasmic side, and since it is a helix protein, some parts would be exposed and some would not. This would explain both predictions of both sources of information.

Figure 5. Predicted protein overview results from Stockholm Bioinformatics Center SBC Phobius.
As a “confirmation” for the previous hypothesis made, Predict Protein site gave the following result:
Figure 7.“Predicted localization for the Bacteria domain: Inner Membrane (GO term ID: 0005886) Prediction confidence 76.”
Based on the predicted result and with the previous hypothesis the team formulated, it was concluded that the protein would act in the cytoplasmic side of the inner membrane. This information was used for the understanding and explaining of the module, as well as for designing different animations.
Further information about NhaS and the experimental results made by the team about it can be found in its parts registry section.
Device
The sequence of the capture device is composed by an UV Promoter (BBa_I765001) was chosen to begin the circuit in order to control the NhaS gene’s expression in E. CARU. The promoter is followed by an RBS (BBa_B0034), the NhaS gene (BBa_K1255000), then the same RBS, an RFP reporter (BBa_E1010) and a terminator (BBa_B1002).
However, in an experimental way it was separated
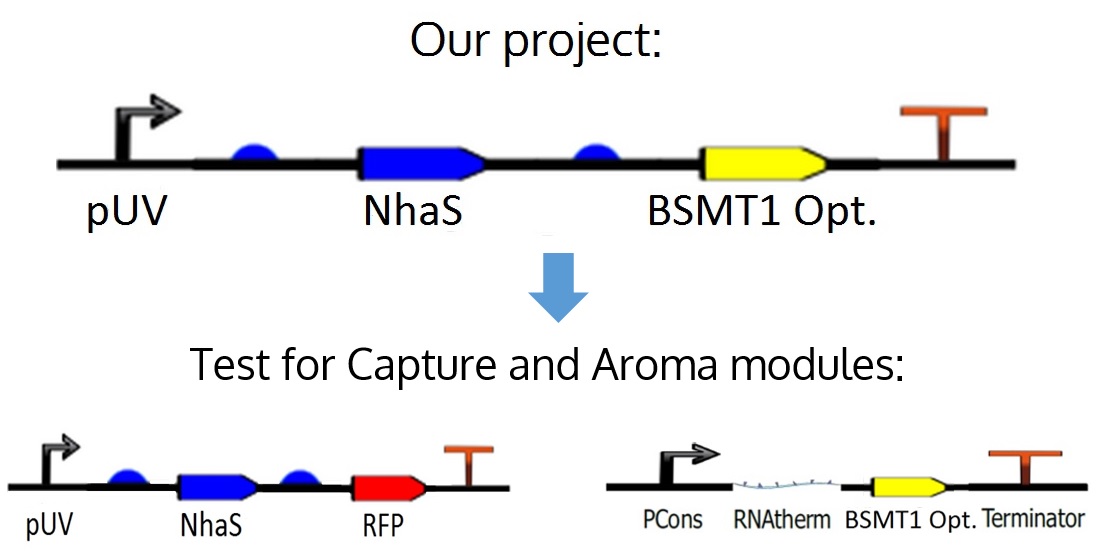
UV promoter as an initiator for the system
|
|
The NhaS’ production and functionability was regulated using a promoter from iGEM Colombian Team 2007 that is regulated by UV irradiation and does not causes mutations in the bacteria. This type of promoter was chosen in order to have a control about the NhaS action in the project, so it would only be activated under UV rays, and therefore, it could be decided at which time the NhaS gene was going to be expressed. There were chosen UV rays as the initiator of the expression because they are present in normal conditions, and in case the biofilter was used in a water treatment plant, as it is intended as a future projection for the project, it could be simpler and cheaper to activate the system with sunlight, since the mentioned promoter is activated under 360 wavelengths of light spectrum. As an extra, the activation of the system without the use of reactants that could pollute the water is one of the advantages of its use. |
Other teams that used pUV
Before choosing the BBa_I765001 UV promoter the team reviewed the experiences by other participants with it, with the intention of assuring the maximum possible success rate if used in this module. The following table shows information about its usage with other teams.
|
Team |
Use |
|
An UV induced promoter and a CDS of a testosterone-making gene.
|
|
|
|
pUV and mCherry (BBa_J06702) to detect DNA damage.
|
|
|
Expression of the UV promoter in presence of UV irradiation light lead to the expression of the EYFP reporter. |
How to know there is any production?
In the ideal project of iGEM CIDEB 2014 it is proposed an odor Wintergreen (BSMT1 Opt.) reporter for this module in order to know if there is any production of NhaS, but since BSMT1 Opt. wasn't probed yet and NhaS is a putative protein it was decided to test it physically with RFP, which is a simple and common reporter, in order to observe functional results in the tests for this specific segment of the project and obtaining conclusions about each one. Further information can be found in the Aroma module in this wiki.

Other teams that used NhaS and potential future uses
There are no teams that used this gene in the past. IGEM CIDEB 2014 was the first team that synthesized, tested and registered it in the parts registry.
The team considers this to be useful for the scientific community in general. NhaS, as mentioned before, is a putative protein. Knowing specific results about its functions could be of great use for future projects.
The potential for future usage of this gene is great. As mentioned in the patent from Krulwich and Ivey (1992), the gene encoding NhaS can be introduced into cells to produce desalination bioreactors, can be introduced into plants as a transgene to produce plants that are resistant to sodium, may be used for treatments involving NNa+ /K+ ATPase disorders, e.g., in heart disease, and may be introduced parenterally, preferably orally, to bind to and sequester dietary sodium. The possibilities are wide and promising.
NhaS part’s description
|
IMAGE |
CODE |
DESCRIPTION |
|
|
UV Promoter from iGEM Colombian Team 2007. Its length is 76bp.
Tim ITB_Indonesia 2013 proved that this part worked for them. |
|
|
|
Very common Ribosome Binding Site, based on Elowitz repressilator. Its length is 12bp
.
|
|
|
|
|
Putative protein that we, iGEM CIDEB 2014, are going to introduce to iGEM for the first time. Its length is 207bp. |
|
|
|
Wintergreen-odor enzyme generator, used to allow the production of methyl salicylate, when it is induced by salicilyc acid. Its length is 1074bp. |
|
|
Part made of 6pb responsible for stopping transcription.
|
Capture Module Zoom In
Bibliography/References
● Antiquity. (2013, January 31). Part: BBa_B0034. Retrieved May 1st, 2014, from http://parts.igem.org/wiki/index.php?title=Part:BBa_B0034
● BMM Cancer Research UK. Interactive 3D Jigsaw. Retrieved May 1st, 2014, from bmm.cancerresearchuk.org
● Colombia Israel Team. (2007). Part: BBa_I765001. Retrieved April 1st, 2014, from Part-BBa_I765001
● IGEM Colombian Team. (2007). UV promoter. Retrieved April 1st, 2014, from http://parts.igem.org/Part:BBa_I765001
● Knight Lab. (2006). Part: BBa_B1002. Retrieved from http://parts.igem.org/Part:BBa_B1002
● KRULWICH Terry, IVEY Mark.(1992) Sodium ion binding proteins. Retrieved April 1st, 2014, from http://www.google.com.mx/patents/US5346815
● Stockholm Bioinformatics Center SBC (2014). Phobius. Retrieved from http://phobius.sbc.su.se
● Predict Protein. Request ID: 473277. Retrieved May 3rd, 2014, from https://www.predictprotein.org
● Raptor X (2014). Protein Structure and Function Prediction: Result for job NhaS. Retrieved June 18th, 2014, from http://raptorx.uchicago.edu/StructurePrediction/myjobs/30991450_67869/
 "
"


















