Team:WalthamHS BioHawks/Project/Design
From 2014hs.igem.org
| Line 1: | Line 1: | ||
<div id="header">{{Team:Waltham_BioHawks/WalthamHeader}}</div> | <div id="header">{{Team:Waltham_BioHawks/WalthamHeader}}</div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 18:12, 20 June 2014
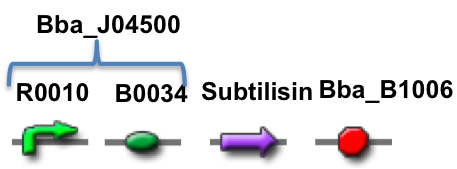
Our stain remover device is built of four main components; a promoter, ribosome binding site (RBS), the subtilisin enzyme and a terminator. The promoter and RBS are taken from the distribution kit under part Bba_J04500. We had the subtilisin enzyme and terminator synthesized by the company BioBasic at reasonable cost. In addition, they synthesized the Biobrick prefix and suffix with the part to make it 3A compatible.
Basic Descriptions:
The promoter and RBS are together in BioBrick Part BBa_J04500, which is the same part used in the practice 3A assembly kit. Subtilisin is a serine protease enzyme that can degrade other proteins. The terminator is BBa_B1006.
Parts Explained: The promoter, R0010, is taken from the inducible lac operon. A lac operon is a series of genes that code for proteins that digest lactose. However in our experiment, those genes have been replaced by genes that code for subtilisin. LacI codes for a repressor that halts transcription by preventing RNA polymerase from binding. In the presence of lactose, the repressor is removed and RNA polymerase is able to bind. IPTG is a molecular mimic of lactose, and therefore in the presence of IPTG, the lac operon operon is turned on. In addition, the lac operon is sensitive to catabolite activator protein, or CAP. CAP is a transcriptional activator and is only functional when glucose levels are low and consequently cAMP levels are high. Therefore the lac operon, and in our case the promoter, is turned on when glucose levels are low and IPTG levels are high.
B0034 is ribosome binding site on mRNA, which facilitates translation.
Before the promoter there is a BB-prefix and after the termination sequence there is a BB-suffix. The two BB sequences are restriction sites where the the gene can be cut and then ligated into a plasmid. The promoter and RBS together are called Part A (BBa_J04500), totaling to 220 base pairs. The specific subtilisin enzyme (Subtilisin BPN’, UniProt ID P00782 6 ) we used has been commercially marketed by the company Novozymes under the name Alcalase for use in detergents. Based on the work of Ghasemi (2012) and Yamabhai (2008) we knew that to get E. coli to excrete our enzyme a signal peptide would have to be added. After our advisor consulted with colleagues, the OmpA signal peptide was chosen. OmpA signal peptide is 21 amino acids long. It helps secrete recombinant enzymes out of the bacteria, where time is an important factor in how much is secreted. Induction time requires several hours, 4 in the experiment by Yamabhai. Normally, it is used to translocate and direct enzymes across the cell membrane of the bacteria. The type of bacteria should not be a major factor, but should also not be ignored. For some enzymes tested by Yamabhai, OmpA was equally effective in secretion as the native signal peptides, however this is not guaranteed for all enzymes. Due to the large variety of enzymes that OmpA can direct, it is advantageous to use it for non-native enzyme secretion in transformed E. coli bacteria.
Subtilisin is a protein-degrading enzyme. The peptide bonds are degraded with serine residue at the active site. The enzyme works in a charge-relay network, using Asp-32, His-64, and Ser-221. The three amino acids form a group in the 3D shape of the protein as is indicated in Figure 1. Ser-221 is able to cleave the peptide bond of proteins with its partially negative oxygen atom as is shown in Figure 2. In commercial use, a genetically engineered form of subtilisin is used, as harsh chemical detergents and high temperatures easily inactivate the wild type. It usually consists of 269 to 275 amino acids and its active from pH 6 to 11, with its major activity from pH 9 to 11. Subtilisin comes from the Bacillus genus and is easily found in the environment (in soil bacteria) and is readily biodegradable.
 "
"