Team:Shenzhen SZMS/Modelling
From 2014hs.igem.org
Peiwen cai (Talk | contribs) (Created page with "==Modeling== In order to assess the efficiency of our E.coli babysitter, we have made math models for the core system in it, EC 1.7.7.2, which functions as to heat up the environ...") |
|||
| (9 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | == | + | {| class="wikitable" align=center |
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS|Home]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/Team Members|Team Members]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/Project|Project]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/Modelling|Modelling]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/Lab|Lab]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/iGem Safety Q&A|Safety]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS/Gallery|Gallery]] | ||
| + | |align=center width=12.5%|[[Team:Shenzhen SZMS#Human Practice|Human Practice]] | ||
| + | |} | ||
| + | |||
| + | [https://2014hs.igem.org/Main_Page<br> <<Back to wiki main page] | ||
| + | ---- | ||
In order to assess the efficiency of our E.coli babysitter, we have made math models for the core system in it, EC 1.7.7.2, which functions as to heat up the environment to provide a more suitable temperature for the plant. | In order to assess the efficiency of our E.coli babysitter, we have made math models for the core system in it, EC 1.7.7.2, which functions as to heat up the environment to provide a more suitable temperature for the plant. | ||
EC 1.7.7.2 is basically a ferredoxin-nitrate reductase; with the required substrates nitrate and reduced ferredoxin, EC 1.7.7.2 is able to catalyze the reactions below: | EC 1.7.7.2 is basically a ferredoxin-nitrate reductase; with the required substrates nitrate and reduced ferredoxin, EC 1.7.7.2 is able to catalyze the reactions below: | ||
| - | nitrite + H<sub>2</sub>O + oxidized ferredoxin ⇄ nitrate + reduced ferredoxin + | + | nitrite + H<sub>2</sub>O + oxidized ferredoxin ⇄ nitrate + reduced ferredoxin + 2H<sup>+</sup> |
| - | HNO<sub>2</sub> + NH<sub>3</sub> = N<sub>2</sub>↑+ 2H | + | HNO<sub>2</sub> + NH<sub>3</sub> = N<sub>2</sub>↑+ 2H<sub>2</sub>O |
obviously as suggested above,the first reaction is a reversible one; the heat we need is actually from the second reaction since the first one is basically to convert nitrate provided by our special LB into nitrite.On the other hand, the warmth released by the first reaction is so little that it has nearly no effect on the result. Consequently,we make only the math models for the second reaction, whose substrate is nitrate. As calculated, the general warmth that would be released is 334.55 KJ/mol, which is a theoretical datum. | obviously as suggested above,the first reaction is a reversible one; the heat we need is actually from the second reaction since the first one is basically to convert nitrate provided by our special LB into nitrite.On the other hand, the warmth released by the first reaction is so little that it has nearly no effect on the result. Consequently,we make only the math models for the second reaction, whose substrate is nitrate. As calculated, the general warmth that would be released is 334.55 KJ/mol, which is a theoretical datum. | ||
| Line 12: | Line 24: | ||
However,considering the fact that we do not have adequate data to carry out our math models, we finally decide to use proportionality instead of accurate data. Both of the following charts are established by Excel. | However,considering the fact that we do not have adequate data to carry out our math models, we finally decide to use proportionality instead of accurate data. Both of the following charts are established by Excel. | ||
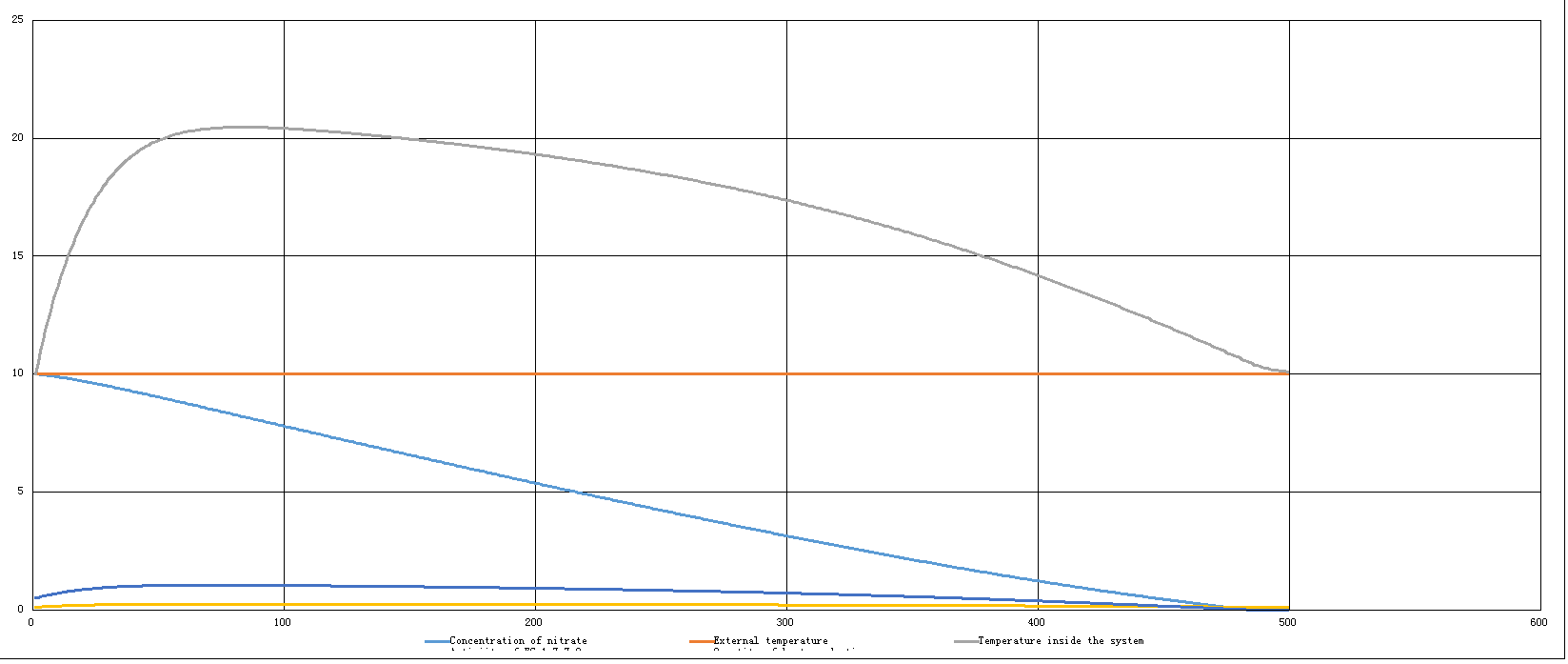
| - | The first one is a model under the condition that purely only EC 1.7.7.2 is functioning, which means, the concentration of it remains unchanged: (grey | + | The first one is a model under the condition that purely only EC 1.7.7.2 is functioning, which means, the concentration of it remains unchanged: (grey line → temperature inside the systerm) |
[[File:nongdumeiyoubianlatest.png|800px]] | [[File:nongdumeiyoubianlatest.png|800px]] | ||
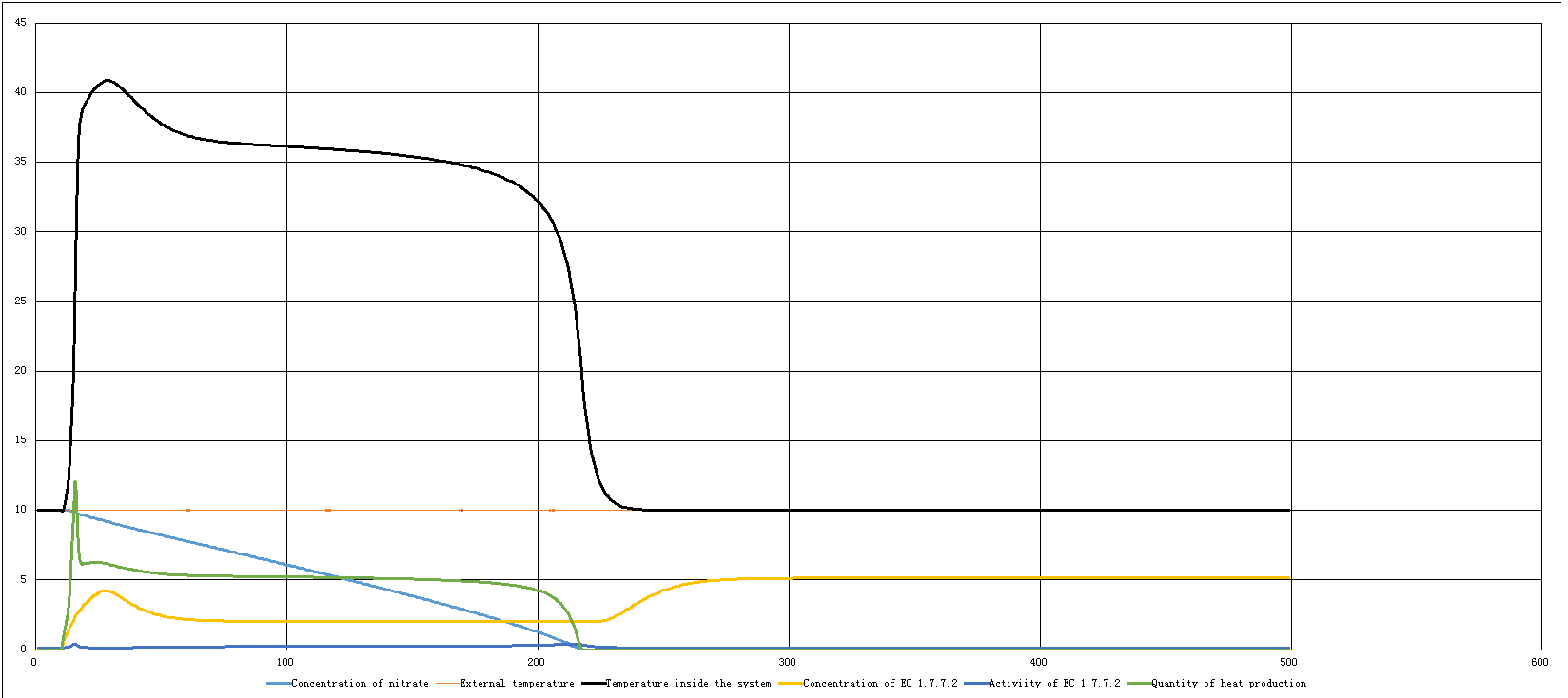
| - | The second one is a model under the condition that RNA Thermometer in the first part of our entire plasmid is functioning to start the heating-up system which is mostly EC 1.7.7.2 in certain extent of external temperature (<27℃). Since it is being manipulated by the other system, the concentration of EC 1.7.7.2 can no long remain unchanged, but changed over time: (black | + | The second one is a model under the condition that RNA Thermometer in the first part of our entire plasmid is functioning to start the heating-up system which is mostly EC 1.7.7.2 in certain extent of external temperature (<27℃). Since it is being manipulated by the other system, the concentration of EC 1.7.7.2 can no long remain unchanged, but changed over time: (black line → temperature inside the systerm) |
[[File:nongdubianleahahaha.png|800px]] | [[File:nongdubianleahahaha.png|800px]] | ||
General information about EC 1.7.7.2 can be found in the following link: http://www.genome.jp/dbget-bin/www_bget?ec:1.7.7.2. | General information about EC 1.7.7.2 can be found in the following link: http://www.genome.jp/dbget-bin/www_bget?ec:1.7.7.2. | ||
Latest revision as of 03:48, 21 June 2014
| Home | Team Members | Project | Modelling | Lab | Safety | Gallery | Human Practice |
In order to assess the efficiency of our E.coli babysitter, we have made math models for the core system in it, EC 1.7.7.2, which functions as to heat up the environment to provide a more suitable temperature for the plant.
EC 1.7.7.2 is basically a ferredoxin-nitrate reductase; with the required substrates nitrate and reduced ferredoxin, EC 1.7.7.2 is able to catalyze the reactions below:
nitrite + H2O + oxidized ferredoxin ⇄ nitrate + reduced ferredoxin + 2H+
HNO2 + NH3 = N2↑+ 2H2O
obviously as suggested above,the first reaction is a reversible one; the heat we need is actually from the second reaction since the first one is basically to convert nitrate provided by our special LB into nitrite.On the other hand, the warmth released by the first reaction is so little that it has nearly no effect on the result. Consequently,we make only the math models for the second reaction, whose substrate is nitrate. As calculated, the general warmth that would be released is 334.55 KJ/mol, which is a theoretical datum.
However,considering the fact that we do not have adequate data to carry out our math models, we finally decide to use proportionality instead of accurate data. Both of the following charts are established by Excel.
The first one is a model under the condition that purely only EC 1.7.7.2 is functioning, which means, the concentration of it remains unchanged: (grey line → temperature inside the systerm)
The second one is a model under the condition that RNA Thermometer in the first part of our entire plasmid is functioning to start the heating-up system which is mostly EC 1.7.7.2 in certain extent of external temperature (<27℃). Since it is being manipulated by the other system, the concentration of EC 1.7.7.2 can no long remain unchanged, but changed over time: (black line → temperature inside the systerm)
General information about EC 1.7.7.2 can be found in the following link: http://www.genome.jp/dbget-bin/www_bget?ec:1.7.7.2.
 "
"