Team:GenetiX Tec CCM
From 2014hs.igem.org
| Line 1: | Line 1: | ||
| - | |||
| - | |||
<html lang="en" dir="ltr" class="client-nojs"> | <html lang="en" dir="ltr" class="client-nojs"> | ||
<head> | <head> | ||
| - | + | <title>GenetiX</title> | |
| - | <title> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<h2 id="Home">Welcome to our wiki</h2> | <h2 id="Home">Welcome to our wiki</h2> | ||
<p>We are high school students at Tecnológico de Monterrey, Mexico | <p>We are high school students at Tecnológico de Monterrey, Mexico | ||
Revision as of 01:09, 21 June 2014
Welcome to our wiki
We are high school students at Tecnológico de Monterrey, Mexico City Campus, a team with juniors and seniors working together. What made us a team was our passion for science, we all wanted to innovate, to create, to surprise and engine something useful. Our goal is to prove that if you plan on doing something, no matter what, you can achieve it with the right focus.
Biodetection of Anoxia in Lake Xochimilco
Lake Xochimilco in Mexico City faces a condition of extreme pollution which endangers the endemic species; many of which are nearing extinction. Oxygen levels depletion in the lake directly affect the fauna, making it less hospitable or even deadly. Our goal is to produce a biosensor that can easily and inexpensively detect anoxia in different regions of the lake. Using an oxygen promoter in addition with the biological markers RFP and GFP we could theoretically detect low dissolved oxygen levels in water samples. In addition, we intend to use a second construct with an Iron promoter to detect iron concentrations that also endanger the sustainability of living organisms in the lake. Once we identify critical regions of the Lake, our report could incentivize the Civil Council and authorities to propose concrete legal initiatives to reduce pollution in the identified areas and start remediation campaigns.
Contents
The Project
The main idea of our project is to achieve the detection of anoxia and Iron concentrations in water systems. What we propose is to construct an easy way of monitoring the levels of O2 and Fe in the lake by using biosensors. By using modified bacteria E. coli for this, we will try to find a cheaper, easier, and faster way to detect the problem of anoxia and iron concentration in some aquifers of Mexico City. We will have to analyze samples of water at different depths to know where the problem is worse and what probable native species could be more affected.
Our purpose is the identification of adequate dissolved oxygen
levels for a stable support of life and the identification of iron
concentration below threatening levels in order to know if the
lake has the physical and chemical properties to support wild-life
naturally. With the use of biosensors, specialized for detecting
concentrations of oxygen above a 2% dissolution, we used modified
E. coli with an oxygen promoter (BBa_K258005) that will
detect the low concentrations and a reporter of (GFP or RFP) that
will indicate the activation of our promoter (Figure 1).
The Iron promoter reacts inside an environment with a
concentration of iron ranging from 1 ppm and on (A.
Quintero,2007). The acceptable levels of iron in drinkable water
are lower than 0.5 ppm (WHO, 1996).

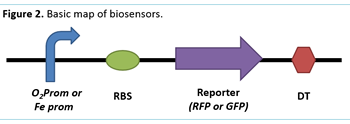
To achieve the objective using E. Coli we will construct different types of modified plasmids for our bacteria to express the biosensors based on iGem biobricks. The idea is to use sensitive promoters: one for oxygen, and another one for iron; those promoters will lead to an expression of GFP or mRFP. This will provide a visual signal to indicate the presence or absence of these elements. Biobrick parts BBa_K258005 (O₂ prom), BBa_I765000 (Fe prom), BBa_E1010 (GFP) and BBa_J04650 (mRFP) were selected for the construction of the biosensors (Figure 2). These were transformed into E. coli strands DH5-a, TOP 10, and NEB 10-b for storage and subsequent plasmid growth and isolation using a Zymo Research® DNA extraction kit.
Once we measure and identify critical regions of the Lake, our report could go directly to the Citizen Council for its consideration. The competent authorities should be able to propose concrete legal initiatives to reduce pollution in the identified areas and start remediation campaigns that re-establish the local aquatic environment to a stable, liveable, friendly ecosystem for the inhabitant species.
Biosensor
A biosensor is an instrument for the measurement of biological or chemical parameters. They usually combine biological and physical-chemical components.
Generally, they consist of three parts:
- The biological sensor: It may be a tissue, a culture of microorganisms, enzymes, antibodies, nucleic acid chains, etc. The sensor can be taken from the wild or be a product of synthetic biology.
- The transducer: Its function is to bind the other two elements and translate the signal emitted by the sensor.
- The detector: It can be optical, piezoelectric, thermal, magnetic, etc.
The most common example is a biosensor that measures blood glucose. It uses an enzyme that processes glucose molecules, releasing an electron for each molecule. Said electron is collected at one electrode and the electron flow is used as a measure of the glucose concentration.

The caged canaries used by miners to detect the presence of lethal
gases can be seen as an early example of biosensors (Wikipedia,
2014)
By using modified bacteria E. coli for this, we will try to find a
cheaper, easier, and faster way to detect the problem of anoxia
and heavy metals in some aquifers of Mexico City. We will have to
analyze samples of water at different depths to know where the
problem is worse and what probable native species could be more
affected.
Some of the benefits of using biosensors instead of other sensing
methods, as observed by Ajit Sadana and AzoSensors, are:
- A fast response in time.
- Fast and continuous measurement.
- High specificity because of its shape-specific recognition.
- Simplicity in its use.
- Capability of measuring concentrations ranging from 10-18 to 10-19 M, so we need low sample requirements.
- Capability of real time measurements.
Eutrophication
Eutrophication is the process by which the increased availability
of one or more limiting growth factors needed for photosynthesis
cause excessive plant and algal growth. Some of these factors are
the amount of carbon dioxide, sunlight and nutrient fertilizers.
The elements coming from the nutrient fertilizers that especially
affect the photosynthesis rate are nitrogen and phosphorus.
(Chislock , 2013)
Plants require many different nutrients or components for the
realization of photosynthesis. Nitrogen and phosphorus are the
first components depleted in the water even though there is a
greater amount of other needed substances. While performing
photosynthesis about 8 times more nitrogen is needed than
phosphorus. Thus, phosphorus limits eutrophication if nitrogen is
more than 8 times abundant as phosphorus, while nitrogen is the
limiting factor when its concentration is less than 8 times
abundant as phosphorus. Erosion of surrounding areas is also an
important cause of eutrophication because the nutrients of the
ground are not retained by the roots of plants and trees that
should be there. So deforestation is an environmental element that
strongly affects this process. (UNEP, 1)
The process of eutrophication of an aquifer occurs naturally over
centuries as they are filled with sediments, abundant in nutrients
(figure 1). However, this process has been recently much
accelerated due to the contamination produced by human activities.
The discharges into aquatic systems bring a lot of limiting
nutrients for eutrophication, including nitrogen and phosphorus.
These polluting human residues thrown up into water systems come
from point and non-point pollution sources. (Chislock, 2013)

Figure 1. Natural eutrophication
The term “point source” is referred to
as any single, discernible source from where the polluting agent
is originated, such as a discharge pipe from a factory, sewage
plant. The other term “non-point source” means that the pollution
does not come from a single determinate source. This type of
pollution happens when water moves across the land and pick in its
way human-made pollutants that can be deposited later on in water
bodies. (Harvey, 1)
There are different levels of eutrophication according to how
severe or advanced the process is. The first and harmless
classification of eutrophication is the oligotrophic, where there
is a low concentration of nutrients in the water and thus less
biologic production. Then we have the mesotrophic where there are
intermediate levels of nutrient concentrations and there is a
moderate biologic production that doesn´t affect severely the
aquatic environment. The real problem begins when we get to the
eutrophic level where there is an elevated concentration of
nutrients and a very high biologic productivity. Another
classification is reserved for where the nutrient levels reach
extremely dangerous concentrations that take the aquifer´s
condition to a critical state; it is called hypertrophic and is
almost always caused by the cultural eutrophication. An important
indicator for the eutrophication level is chlorophyll. The total
amount of chlorophyll represents about 1% of plant biomass, so in
this way the total biomass can be estimated allowing the
determination of the degree of eutrophication. (Mazzeo, 1)

Table expressing the characteristic values for each of the
eutrophication classifications. (UNEP)
Eutrophication brings a lot of complications to aquifers. The
enormous creation of dense blooms of noxious, foul-smelling
phytoplankton reduces water clarity and harms water clarity. These
blooms limit light penetration to the water body. This limiting of
sunlight to littoral zones causes the die-offs of the great amount
of plants and algae that grew up without control due to
eutrophication. When these dense algal blooms eventually die,
microorganisms start the decomposition of organic matter and
severely deplete the available dissolved oxygen, causing hypoxia
or even anoxia. These hypoxic environments are cause of dead zones
for most of the inhabiting organisms for the lacking oxygen.
(Chislock 2013)
The normal levels of dissolved oxygen in water for the maintenance
of life are around 6mg/L. Environments are considered hypoxic when
the concentration of dissolved oxygen goes below 2.8 mg/L. When
the dissolved oxygen levels reach the hypoxic condition many
species die. Depending on the size and other characteristics of
the organisms, the limiting concentration for survival will have
low variations. The hypoxic conditions can change in different
lapses of time. They can occur just for a few moments
(minutes/hours) or they can reach chronic states that last for
weeks or even months, causing depletion of local species.
(Cisterna 2008)
It is important to supervise aquatic environments conditions´ to
prevent the initiation of eutrophic conditions. Eutrophication can
kill all life in natural environments. If some symptoms of
eutrophication are detected in time it is possible to attack the
problem and control it, or even eliminate it. Some methods for
controlling eutrophication are:
- Covering sediments, preventing release of nutrients.
- Biomanipulation
- Using chemicals such as copper sulfate to kill excess of algae
- Aerating the hypolimnion of a lake, reducing the release of nutrients from the sediments.
(UNEP)
Localization
First of all, we have to know what eutrophication is, well the eutrophication is the process of excessive growth of algae and weeds water in the water, caused by phosphates and other pollutants discharged to waters. In a eutrophic aquatic ecosystem two things happen: more oxygen is required to break down and increases the population of organisms known as primary producers: organisms that make photosynthesis, as macroalgae and lilies. These can reach atrophy processes exchange of oxygen and water flow. The liquid is cloudy and the lack of oxygen can devastate populations of various organisms.
How does it affect the lake of Xochimilco?
In hydrological Xochimilco area that is located south of the metropolitan area of Mexico City's 189 miles of canals that have been contaminated by the contribution of sewage, domestic waste, industrial, agricultural and drainage system leaks. This has affected different species of living beings that inhabit the area, causing a decline in biodiversity (Juárez-Figueroa et al., 2003).It is estimated that close to urban centers or agricultural nutrient input to a lake can be accelerated by the activities human, a process known as cultural eutrophication, which is the case with Lake Xochimilco to be within the city of Mexico. This is precisely effluent mainly caused by plants sewage treatment, containing nitrates and phosphates runoff of fertilizers and waste animals and accelerated erosion of eutrophication .The eutrophication derived from crops by the recent addition of phosphates and nitrates, as a result of activities human, it is also a serious problem for lakes, especially Lake Xochimilco. During warm periods, the overhead produce dense growth nutrient vegetables such as algae, cyanobacteria, water lilies and duckweed. Oxygen dissolved in the surface layer of water is near die exhausted when large masses of algae, which fall to the bottom and are decomposed by aerobic bacteria. This can kill fish and other animals water they consume oxygen. If theexcess nutrients continue to flow a body of water, the water reaches the bottom be rotten and almost unusable for living things, because bacteria take over and produce anaerobic decomposing substances with poor odors, such as hydrogen sulfide and the methane.
Factors
The factors affecting the degree of eutrophication are:
- Climate: warm climates favor the process.
- Shallow bodies of water and / or low flow are more conducive to the development process
- Drainage Area: little tree cover subject to abundant rainfall tions favors erosion and entrainment of nutrients into the water body
- Geology: In drainage areas where sedimentary rocks predominate vapor no greater phosphorus runoff. Clay soils drain poorly and also favor runoff and result in nutrient supply.
The causes of eutrophication include:
a) Natural:
- atmospheric inputs: precipitation.
- resuspension of bottom sediments.
- release from anoxic sediments.
- breakdown and excretion of organisms.
- nitrogen-fixing microorganisms.
b) Anthropogenic:
- Discharges of industrial, agricultural, municipal and waste treatment plants.
- Deforestation increases erosion and reduces the recycling of nutrients in the watershed, increasing their income to the water body.
- Fertilizer applied in excess.
- Sewage farms (silos, drums).
- Septic tanks.
- Detergents with large amounts of phosphorus.
- Contribution of pollutants from rainwater.
- Sewer system of cities and towns do.
- Measures to control eutrophication include:
Control of nutrient inputs:
- Waste treatment before being poured into the body of water.
- Restricting the use of phosphate detergents.
- Control of land use.
- Prepantanos: remove nutrients from waste water that are fixed in the biomass of algae and macrophytes.
- Physical and chemical waste water treatment: chemical precipitation and filtration.
What we found....
Elements found in the lake of Xochimilco
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| MO mg/LO | 154 | 251 | 88 | 151 | 555 | 295 | 25 | 75 |
| Phosphorus mg/L | 5.8 | 5.6 | 7.6 | 6.7 | 23.0 | 17.1 | 15.0 | 14.2 |
| pH | 6.2 | 7.2 | 6.7 | 7.6 | 8.0 | 7.0 | 7.0 | 7.0 |
| Temperature °C | 3.8 | 3.8 | 4.8 | 3.8 | 6.9 | 7.0 | 7.0 | 7.0 |
| Precipitation | 21.0 | 20.0 | 22.0 | 23.0 | 21.5 | 19.8 | 21.0 | 54 |
| MO = Organic Mater |
 "
"



